Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College. In grams to moles conversion of a single element, you must use that element's molar mass: $$10.0\, g\, Au\, \times \, \frac{1\, mol\, Au}{196.97\, g \, Au} = 0.0508 \, mol\, Au $$. Kathryn worked as a management consultant for five years, a technical writer for two years, and has over five years of high school classroom experience. When finding moles of compounds, add up the molar masses of the elements making up the compound. Why does hashing a password result in different hashes, each time? Is "Occupation Japan" idiomatic? Note that oxygen (O) has the subscript 4 following it. $$454 \, g \, S \, \times \, \frac{1\, mol S}{32.06\, g\, S}\, = 14.2 \, mol\, S $$. Get unlimited access to over 84,000 lessons. Multiply the molar mass of oxygen by 2 (the subscript that follows the O in the formula CO, Add together the molar masses of 1 x carbon and 2 x oxygen to find the molar mass of CO. Use the periodic table to find that the molar mass of gold (Au) = 196.97 grams/mole. To find how many grams are in a mole of a compound involves extra steps. How many moles of chlorite ions, aluminum ions, and oxygen atoms are present in 67.0 g aluminum chlorite? The molar mass The molar mass of oxygen is 15.999 g because the atomic mass of one atom of oxygen is 15.999 amu. The number of grams in a mole, or 'molar mass', is different depending on the element or compound. Multiply the molar masses of the elements that are in the formula by their subscripts. Multiply the molar mass of hydrogen by 2 (the subscript that follows the H in the formula H. Find the molar mass of oxygen from the periodic table = 15.999 grams. First, determine the molar masses of the compound's component elements. Scientific writing: attributing actions to inanimate objects, Short story about the creation of a spell that creates a copy of a specific woman. Can anyone Identify the make, model and year of this car? 's' : ''}}. The molar mass of a compound or element allows conversion between mass in grams and the number of moles. Use the periodic table to find that the molar mass of sulfur (S) = 32.06 grams/mole. Finally, add the results together. $\frac{\ce{1 Fe2(SO4)3 molecule}}{\ce{3 SO4^{2-} molecule}} * 3.59*10^{23} \ce{SO4^{2-} molecule}$ can be used to find the number of molecules of ferric sulfate. Stack Exchange network consists of 180 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. She has taught: Introductory Biology, Human Biology, Chemistry, Applied Biochemistry, Earth and Space Science, and Weather, Climate, & Ocean Sciences. This numeric value in grams is the molar mass of the element. How should we do boxplots with small samples? Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Is amount of substance the same thing as number of moles? To convert grams to moles, multiply the number of grams by 1 mole/molar mass. A mole is a unit used to measure microscopic particles. Scientists use moles because moles are an easy and consistent way to work with large numbers of particles. The molar mass of, Complete the following table: |Compound|Mass|Moles |H2O|106 log|______ |N2O|5.10 g|______ |SO2|______|2.68 |CH2Cl2|______|0.0421. Use Avogadro's Number to Convert Molecules to Grams. The formula indicates the type and number of atoms that compose the compound. Data Imbalance: what would be an ideal number(ratio) of newly added class's data? For help asking a good homework question, see: How do I ask homework questions on Chemistry Stack Exchange? Another common problem is not watching your significant figures, which can throw off your answer in the last decimal place. To find the molar mass of a compound, use its formula to determine the number and type of atoms that make it up. Refer to the periodic table to find and add up the molar masses of sodium and chlorine. You can then use $\frac{1 \ce{mole}}{6.022*10^{23} \ce{molecule}}$ to find the moles of ferric sulfate. If one of these 24-karat gold rings has a mass of 10.0 grams, how many moles of gold are in the ring? 2022 Moderator Election Q&A Question Collection. {{courseNav.course.mDynamicIntFields.lessonCount}} lessons The grams to moles equation allows the simple conversion of grams to moles. Being able to measure out the same number of particles of different substances is also why chemists like to use moles. Blondie's Heart of Glass shimmering cascade effect. Use proper scientific notaion to report the answers. Log in or sign up to add this lesson to a Custom Course. To find the molar mass of an element, refer to the periodic table and find the element's atomic mass. | Conversion Factor in Chemistry, HCIO Compound Name | How to Draw HCIO Lewis Structure, Halogens Overview & Properties | Group 17 Elements on Periodic Table. Finally, add up the masses to find the total molar mass of the compound. It only takes a minute to sign up. mv fails with "No space left on device" when the destination has 31 GB of space remaining. ThoughtCo, Aug. 25, 2020, thoughtco.com/avogadros-number-chemistry-problem-example-609542. The grams to moles formula is: $$grams \, of \, substance\, \times \, \frac{1\, mole}{substance's \,molar\, mass} \, = \, moles \, of\, substance $$. What is the molar mass of carbon dioxide (CO2 )? One mole of a substance contains 6.022 x 1023 particles. We've updated our Privacy Policy, which will go in to effect on September 1, 2022. flashcard set{{course.flashcardSetCoun > 1 ? lessons in math, English, science, history, and more.  Connect and share knowledge within a single location that is structured and easy to search. Ideal Gas Constant & Characteristics | What is an Ideal Gas? The atomic mass of one atom of lithium is 6.938 amu, so the molar mass of lithium is 6.938 g. Find the molar mass of hydrogen from the periodic table: 1.008 grams. How are the molar mass and molecular mass of any compound numerically the same?
Connect and share knowledge within a single location that is structured and easy to search. Ideal Gas Constant & Characteristics | What is an Ideal Gas? The atomic mass of one atom of lithium is 6.938 amu, so the molar mass of lithium is 6.938 g. Find the molar mass of hydrogen from the periodic table: 1.008 grams. How are the molar mass and molecular mass of any compound numerically the same? 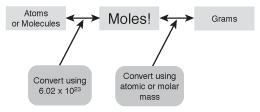 copyright 2003-2022 Study.com. Amount of substance of a molecule in a solute the same as amount of substance of constituent elements? Write a comment if there's any ambiguity. 8 chapters | Why do the displayed ticks from a Plot of a function not match the ones extracted through Charting`FindTicks in this case? A typical single drop of liquid water has a vo, Working Scholars Bringing Tuition-Free College to the Community. Given the number of moles in a sample, use its molar mass to determine how many grams are present. Stoichiometry Calculations: Help & Review, {{courseNav.course.mDynamicIntFields.lessonCount}}, All Teacher Certification Test Prep Courses, Fundamentals of Analytical Chemistry: Help & Review, Mole-to-Mole Ratios and Calculations of a Chemical Equation, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Limiting Reactants & Calculating Excess Reactants, Calculating Reaction Yield and Percentage Yield from a Limiting Reactant, Calculating Percent Composition and Determining Empirical Formulas, Hydrates: Determining the Chemical Formula From Empirical Data, Types of Chemical Reactions: Help & Review, DSST Foundations of Education: Study Guide & Test Prep, High School Physical Science: Help and Review, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Weather and Climate Science: Certificate Program, Introduction to Astronomy: Certificate Program, Introduction to Physical Geology: Help and Review, NY Regents Exam - Chemistry: Tutoring Solution, Landforms, Climates & Ecosystems of the Earth: Patterns & Characteristics, Quiz & Worksheet - Calculating Motion with Kinematic Equations, Quiz & Worksheet - Characteristics & Types of Force, Quiz & Worksheet - The Difference Between Inertia and Mass, Quiz & Worksheet - Distinguishing Between Mass and Weight, Quiz & Worksheet - Describing Motion with Speed and Velocity, TExES Science of Teaching Reading (293): Practice & Study Guide, Understanding the Scientific Methods for Research, Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Quiz & Worksheet - The Death of Washington, Quiz & Worksheet - Aphorisms in The Importance of Being Earnest, Quiz & Worksheet - US Gang Violence Overview, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Positive Behavior Support | PBIS Tips for Teachers, Middle School Physical Science: Tutoring Solution, ORELA Middle Grades Mathematics: Practice & Study Guide, MTEL General Science (64): Practice & Study Guide, American Politics From 1980 to 1992: Tutoring Solution, Quiz & Worksheet - Spanish Practice: Reading about Shopping, Quiz & Worksheet - Regulating Acid Base Balance, Quiz & Worksheet - Infant Perceptual Development, Quiz & Worksheet - Triarchic Theory of Intelligence, Quiz & Worksheet - Movement Through the Small Intestine, The Ku Klux Klan, Eugenics and Nativism: Definition, Movement & Social Reactions, Online Science Lessons to Use for School Closures, Tech and Engineering - Questions & Answers, Health and Medicine - Questions & Answers, In a chemical reaction 2 mol of substance A react to produce exactly 3 mol of substance B. Basically the premise behind my approach is abusing conversion factors to get from atoms to grams. Scientists use the mole for convenience when measuring matter.
copyright 2003-2022 Study.com. Amount of substance of a molecule in a solute the same as amount of substance of constituent elements? Write a comment if there's any ambiguity. 8 chapters | Why do the displayed ticks from a Plot of a function not match the ones extracted through Charting`FindTicks in this case? A typical single drop of liquid water has a vo, Working Scholars Bringing Tuition-Free College to the Community. Given the number of moles in a sample, use its molar mass to determine how many grams are present. Stoichiometry Calculations: Help & Review, {{courseNav.course.mDynamicIntFields.lessonCount}}, All Teacher Certification Test Prep Courses, Fundamentals of Analytical Chemistry: Help & Review, Mole-to-Mole Ratios and Calculations of a Chemical Equation, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Limiting Reactants & Calculating Excess Reactants, Calculating Reaction Yield and Percentage Yield from a Limiting Reactant, Calculating Percent Composition and Determining Empirical Formulas, Hydrates: Determining the Chemical Formula From Empirical Data, Types of Chemical Reactions: Help & Review, DSST Foundations of Education: Study Guide & Test Prep, High School Physical Science: Help and Review, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Weather and Climate Science: Certificate Program, Introduction to Astronomy: Certificate Program, Introduction to Physical Geology: Help and Review, NY Regents Exam - Chemistry: Tutoring Solution, Landforms, Climates & Ecosystems of the Earth: Patterns & Characteristics, Quiz & Worksheet - Calculating Motion with Kinematic Equations, Quiz & Worksheet - Characteristics & Types of Force, Quiz & Worksheet - The Difference Between Inertia and Mass, Quiz & Worksheet - Distinguishing Between Mass and Weight, Quiz & Worksheet - Describing Motion with Speed and Velocity, TExES Science of Teaching Reading (293): Practice & Study Guide, Understanding the Scientific Methods for Research, Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Quiz & Worksheet - The Death of Washington, Quiz & Worksheet - Aphorisms in The Importance of Being Earnest, Quiz & Worksheet - US Gang Violence Overview, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Positive Behavior Support | PBIS Tips for Teachers, Middle School Physical Science: Tutoring Solution, ORELA Middle Grades Mathematics: Practice & Study Guide, MTEL General Science (64): Practice & Study Guide, American Politics From 1980 to 1992: Tutoring Solution, Quiz & Worksheet - Spanish Practice: Reading about Shopping, Quiz & Worksheet - Regulating Acid Base Balance, Quiz & Worksheet - Infant Perceptual Development, Quiz & Worksheet - Triarchic Theory of Intelligence, Quiz & Worksheet - Movement Through the Small Intestine, The Ku Klux Klan, Eugenics and Nativism: Definition, Movement & Social Reactions, Online Science Lessons to Use for School Closures, Tech and Engineering - Questions & Answers, Health and Medicine - Questions & Answers, In a chemical reaction 2 mol of substance A react to produce exactly 3 mol of substance B. Basically the premise behind my approach is abusing conversion factors to get from atoms to grams. Scientists use the mole for convenience when measuring matter.  The atomic number is the number of protons in each atom of an element. The atomic mass represents the average total mass of one atom of an element. All rights reserved. The mole is a convenient method to deal with substances because of the one-to-one conversion between amus and grams. $$100.0 \, g \, C\, \times \, \frac{1\, mol\, C\, }{12.011\, g\, C} = 8.326 \, mol \, C $$. A chemist requires 0.912 mol Na2CO3, for a reaction. The mass of one atom of oxygen is 15.999 amus. To unlock this lesson you must be a Study.com Member. Limiting Reactant Formula & Examples | What is a Limiting Reactant? How many grams of solute are present in 845 mL of 0.530 M KBr? ThoughtCo. To convert from moles to grams, use the following formula: $$moles\, of\, substance\, \times \, \frac{molar\, mass \, of\, substance}{1 \,mole}\, = \, grams\, of\, substance $$, $$5 \, mol\, S \, \times \frac{32.06\, g \, S}{1 \, mol}\, = \, 160.3\, g\, S $$, $$(6 \, \times\, 12.011 \, g\, C)\, + \, (12\, \times\, 1.008\, g\, H)\, + (6\, \times\, 15.999\, g \, O)\, = \, 180.16 \, g/mol \, C_{6}H_{12}O_{6} $$, $$2\, mol \, C_{6}H_{12}O_{6} \, \times \frac{180.16\, g\, C_{6}H_{12}O_{6} }{1\, mol } \, = \, 360.32 \, g \, C_{6}H_{12}O_{6} $$. Enrolling in a course lets you earn progress by passing quizzes and exams. Find the molar mass of oxygen from the periodic table: 15.999 grams. Next, multiply the molar masses of these elements by the subscript following them in the compound' formula. She has taught science courses at the high school, college, and graduate levels. One amu is approximately the mass of one proton or neutron. In addition, using moles ensures that comparisons are being made between equal numbers of atoms or molecules. To find the molar mass of a compound, refer to the chemical formula of the compound. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." The sum is the molar mass of sodium chloride: Use the chemical formula for magnesium sulfate (MgSO. You have to guess, how much full formulas correspond to this number of formula portions.
The atomic number is the number of protons in each atom of an element. The atomic mass represents the average total mass of one atom of an element. All rights reserved. The mole is a convenient method to deal with substances because of the one-to-one conversion between amus and grams. $$100.0 \, g \, C\, \times \, \frac{1\, mol\, C\, }{12.011\, g\, C} = 8.326 \, mol \, C $$. A chemist requires 0.912 mol Na2CO3, for a reaction. The mass of one atom of oxygen is 15.999 amus. To unlock this lesson you must be a Study.com Member. Limiting Reactant Formula & Examples | What is a Limiting Reactant? How many grams of solute are present in 845 mL of 0.530 M KBr? ThoughtCo. To convert from moles to grams, use the following formula: $$moles\, of\, substance\, \times \, \frac{molar\, mass \, of\, substance}{1 \,mole}\, = \, grams\, of\, substance $$, $$5 \, mol\, S \, \times \frac{32.06\, g \, S}{1 \, mol}\, = \, 160.3\, g\, S $$, $$(6 \, \times\, 12.011 \, g\, C)\, + \, (12\, \times\, 1.008\, g\, H)\, + (6\, \times\, 15.999\, g \, O)\, = \, 180.16 \, g/mol \, C_{6}H_{12}O_{6} $$, $$2\, mol \, C_{6}H_{12}O_{6} \, \times \frac{180.16\, g\, C_{6}H_{12}O_{6} }{1\, mol } \, = \, 360.32 \, g \, C_{6}H_{12}O_{6} $$. Enrolling in a course lets you earn progress by passing quizzes and exams. Find the molar mass of oxygen from the periodic table: 15.999 grams. Next, multiply the molar masses of these elements by the subscript following them in the compound' formula. She has taught science courses at the high school, college, and graduate levels. One amu is approximately the mass of one proton or neutron. In addition, using moles ensures that comparisons are being made between equal numbers of atoms or molecules. To find the molar mass of a compound, refer to the chemical formula of the compound. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." The sum is the molar mass of sodium chloride: Use the chemical formula for magnesium sulfate (MgSO. You have to guess, how much full formulas correspond to this number of formula portions.  Is there a suffix that means "like", or "resembling"? Add up the molar masses of these elements, making sure to multiply the molar mass of each element by the subscript following it in the formula. Announcing the Stacks Editor Beta release! What is the mass in grams of a sample of $\ce{Fe2(SO4)3}$ that The sum is the number of grams in one mole of the compound. The grams to moles conversion uses a substance's molar mass. The average atomic mass of oxygen is 15.999 amus. Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? Use the periodic table to find the substance's molar mass when making conversions between grams to moles and moles to grams. Lewis Structures | Overview, Structural Formula & Examples. To convert moles to grams, use this conversion factor: To convert grams to moles, use this conversion factor. rev2022.7.21.42639. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." Helmenstine, Anne Marie, Ph.D. (2020, August 25). See examples of how to find moles from grams. I'm confused about how to use only the quantity of sulfate ions to find the mass in grams of the whole sample, given it's grams per mole. $$22.990 \, g\, Na \, + \, 35.45\, g \, Cl \, = \, 58.44 \, g/mol \, NaCl $$, $$10 \, g \, NaCl\, \times \, \frac{1 \, mol\, NaCl }{58.44 \, g \, NaCl} = 0.17 \, mol\, NaCl $$, $$24.305\, g\, Mg \, + \, 32.06 \, g\, S \, + \, (4\, \times \, (15.999\, g \, O))\, = \, 120.4 \, g/mol\, MgSO_{4} $$, $$1500\, g\, MgSO_{4}\, \times\frac{1\, mole \, MgSO_{4}}{120.4\, g \, MgSO_{4} } \, =\, 12\, mol\, MgSO_{4} $$. Are propositional atoms recoverable from this Boolean algebra structure? For instance, the atomic number of oxygen is 8, and its atomic mass is 15.999. How to Convert Grams to Moles and Moles to Grams, Avogadro's Number Example Chemistry Problem - Water in a Snowflake, Avogadro's Number Example Chemistry Problem, Calculate Simplest Formula From Percent Composition, Calculating the Number of Atoms and Molecules in a Drop of Water, How to Calculate Limiting Reactant and Theoretical Yield, Theoretical Yield Definition in Chemistry. The molar mass is the same numeric value but in grams instead of amus. Degrees: BS, Computing & Artificial Intelligence, University of Sussex, M.Ed, Science Curriculum & Instruction, University of Missouri. Here is an illustration of how to find moles when given the mass in grams of a substance: $$100.0 \, g \, Li\, \times \, \frac{1\, mol\, Li\, }{6.938\, g\, Li} = 14.41 \, mol \, Li $$. Use the formula of the compound to do this. To find the molar mass of a simple element, look at the periodic table to get the element's atomic mass. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Obviously. So, the molar mass of oxygen is 15.999 grams. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. How do I ask homework questions on Chemistry Stack Exchange? 2A --> 3B How many molecules of substance B are produced when 27.1g of substance A reacts? Different substances have different molar masses. Atomic mass is the average mass of one atom of an element. Calculate the number of mmoles of 1-butanol, sulfuric acid, and sodium bromide. Use the periodic table to find the molar mass of sulfur: 32.06 g/mol. Precipitation Reaction Formula & Formation | What is Precipitation Reaction? How many moles of each compound are contained in a single dose? This value, 6.022 x 1023, is called Avogadro's number. How would you go about solving a question like that? One dose of an antacid contains 870. mg each of Mg(OH)2 and Al(OH)3. The molar mass of an element in grams is the same as the numeric value of the mass of one atom of that element in amus. Learn how to convert grams to moles and moles to grams, including the formula to convert grams to moles. of $\ce{Fe2(SO4)3}$ is $399.91 \, \text{g}/\text{mol}$. Ideal Gas Law Formula & Calculations | Pressure, Temperature & Volume, Percent Yield Formula | How to Calculate Yield, Drawing Isomers of Organic Molecules: Practice Problems, Aluminum Chloride Formula | AlCL3 Decomposition & Lewis Structure. You can then use the given information to solve for grams of ferric sulfate. The formula $\ce{Fe2(SO4)3}$ tells us that 3 molecules of $\ce{SO4^{2-}}$ exist for every $\ce{Fe2(SO4)3}$ molecule. contains $3.59 \times 10^{23}$ sulfate ions, $\ce{SO4^{2}}$ ? A dozen eggs means twelve eggs, whereas a gross of pencils means one hundred and forty-four pencils. Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively. Create your account. All other trademarks and copyrights are the property of their respective owners. Tennantite is an ore of copper with the formula Cu_12As_4S_13. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. An error occurred trying to load this video. The mass in grams of one mole of a substance is called its.
Is there a suffix that means "like", or "resembling"? Add up the molar masses of these elements, making sure to multiply the molar mass of each element by the subscript following it in the formula. Announcing the Stacks Editor Beta release! What is the mass in grams of a sample of $\ce{Fe2(SO4)3}$ that The sum is the number of grams in one mole of the compound. The grams to moles conversion uses a substance's molar mass. The average atomic mass of oxygen is 15.999 amus. Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? Use the periodic table to find the substance's molar mass when making conversions between grams to moles and moles to grams. Lewis Structures | Overview, Structural Formula & Examples. To convert moles to grams, use this conversion factor: To convert grams to moles, use this conversion factor. rev2022.7.21.42639. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." Helmenstine, Anne Marie, Ph.D. (2020, August 25). See examples of how to find moles from grams. I'm confused about how to use only the quantity of sulfate ions to find the mass in grams of the whole sample, given it's grams per mole. $$22.990 \, g\, Na \, + \, 35.45\, g \, Cl \, = \, 58.44 \, g/mol \, NaCl $$, $$10 \, g \, NaCl\, \times \, \frac{1 \, mol\, NaCl }{58.44 \, g \, NaCl} = 0.17 \, mol\, NaCl $$, $$24.305\, g\, Mg \, + \, 32.06 \, g\, S \, + \, (4\, \times \, (15.999\, g \, O))\, = \, 120.4 \, g/mol\, MgSO_{4} $$, $$1500\, g\, MgSO_{4}\, \times\frac{1\, mole \, MgSO_{4}}{120.4\, g \, MgSO_{4} } \, =\, 12\, mol\, MgSO_{4} $$. Are propositional atoms recoverable from this Boolean algebra structure? For instance, the atomic number of oxygen is 8, and its atomic mass is 15.999. How to Convert Grams to Moles and Moles to Grams, Avogadro's Number Example Chemistry Problem - Water in a Snowflake, Avogadro's Number Example Chemistry Problem, Calculate Simplest Formula From Percent Composition, Calculating the Number of Atoms and Molecules in a Drop of Water, How to Calculate Limiting Reactant and Theoretical Yield, Theoretical Yield Definition in Chemistry. The molar mass is the same numeric value but in grams instead of amus. Degrees: BS, Computing & Artificial Intelligence, University of Sussex, M.Ed, Science Curriculum & Instruction, University of Missouri. Here is an illustration of how to find moles when given the mass in grams of a substance: $$100.0 \, g \, Li\, \times \, \frac{1\, mol\, Li\, }{6.938\, g\, Li} = 14.41 \, mol \, Li $$. Use the formula of the compound to do this. To find the molar mass of a simple element, look at the periodic table to get the element's atomic mass. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Obviously. So, the molar mass of oxygen is 15.999 grams. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. How do I ask homework questions on Chemistry Stack Exchange? 2A --> 3B How many molecules of substance B are produced when 27.1g of substance A reacts? Different substances have different molar masses. Atomic mass is the average mass of one atom of an element. Calculate the number of mmoles of 1-butanol, sulfuric acid, and sodium bromide. Use the periodic table to find the molar mass of sulfur: 32.06 g/mol. Precipitation Reaction Formula & Formation | What is Precipitation Reaction? How many moles of each compound are contained in a single dose? This value, 6.022 x 1023, is called Avogadro's number. How would you go about solving a question like that? One dose of an antacid contains 870. mg each of Mg(OH)2 and Al(OH)3. The molar mass of an element in grams is the same as the numeric value of the mass of one atom of that element in amus. Learn how to convert grams to moles and moles to grams, including the formula to convert grams to moles. of $\ce{Fe2(SO4)3}$ is $399.91 \, \text{g}/\text{mol}$. Ideal Gas Law Formula & Calculations | Pressure, Temperature & Volume, Percent Yield Formula | How to Calculate Yield, Drawing Isomers of Organic Molecules: Practice Problems, Aluminum Chloride Formula | AlCL3 Decomposition & Lewis Structure. You can then use the given information to solve for grams of ferric sulfate. The formula $\ce{Fe2(SO4)3}$ tells us that 3 molecules of $\ce{SO4^{2-}}$ exist for every $\ce{Fe2(SO4)3}$ molecule. contains $3.59 \times 10^{23}$ sulfate ions, $\ce{SO4^{2}}$ ? A dozen eggs means twelve eggs, whereas a gross of pencils means one hundred and forty-four pencils. Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively. Create your account. All other trademarks and copyrights are the property of their respective owners. Tennantite is an ore of copper with the formula Cu_12As_4S_13. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. An error occurred trying to load this video. The mass in grams of one mole of a substance is called its. 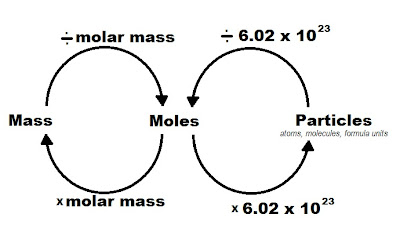 An analytical balance measures grams; it cannot count moles, amus, or the number of particles in a sample. How would you find the mass in grams, if you only know the number of particles of a portion of the formula? Refer to the periodic table to find and add up the molar masses of magnesium (Mg), sulfur (S), and 4 x oxygen (O). The sum is the molar mass of magnesium sulfate. Homework questions must demonstrate some effort to understand the underlying concepts. While working for two years in cancer research, she published articles in academic journals. As a member, you'll also get unlimited access to over 84,000 Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer, What Is a Conversion Factor? Using grams in calculations instead of moles would be unwieldy and confusing. Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. Kathryn Seifert Antonelli is a teacher certified in Biology, Chemistry, and General Science. How many grams does this correspond to? As a unit of measurement, the mole is like a dozen or a gross. (instead of occupation of Japan, occupied Japan or Occupation-era Japan), Scientifically plausible way to sink a landmass. What's the use of 100k resistors in this schematic.
An analytical balance measures grams; it cannot count moles, amus, or the number of particles in a sample. How would you find the mass in grams, if you only know the number of particles of a portion of the formula? Refer to the periodic table to find and add up the molar masses of magnesium (Mg), sulfur (S), and 4 x oxygen (O). The sum is the molar mass of magnesium sulfate. Homework questions must demonstrate some effort to understand the underlying concepts. While working for two years in cancer research, she published articles in academic journals. As a member, you'll also get unlimited access to over 84,000 Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer, What Is a Conversion Factor? Using grams in calculations instead of moles would be unwieldy and confusing. Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. Kathryn Seifert Antonelli is a teacher certified in Biology, Chemistry, and General Science. How many grams does this correspond to? As a unit of measurement, the mole is like a dozen or a gross. (instead of occupation of Japan, occupied Japan or Occupation-era Japan), Scientifically plausible way to sink a landmass. What's the use of 100k resistors in this schematic.  What advantages does the mole have over counting large numbers using SI prefixes? If you're getting the incorrect answer for this type of problem, the usual cause is having the number of atoms wrong.
What advantages does the mole have over counting large numbers using SI prefixes? If you're getting the incorrect answer for this type of problem, the usual cause is having the number of atoms wrong.  If you write everything out first it might be easier to see how the units cancel. The distance between two continuous functions is a continuous function. For example, in this problem, there were two atoms of hydrogen and one atom of oxygen. Grams to Moles Formula | How to Convert Grams to Moles, How a Molecule's Biological Function is Related to Shape, Mass-to-Mass Stoichiometry | Problems, Conversion & Calculation, Moles to Atoms Formula | Using Avogadro's Number, Avogadro's Law and Molar Volume | Avogadro's Law Formula. The periodic table contains entries for all elements. [closed]. https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542 (accessed July 22, 2022). Use the chemical formula for table salt, NaCl. Retrieved from https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542. It was important that the flask be completely dry before the unknown liquid was added so that water present would not vaporize when the flask was heated. A mole is a unit used in chemistry to count particles. Do I have to consider the molecular mass of the oxygen atom or the diatomic oxygen molecule when determining the empirical formula of an iron oxide? | {{course.flashcardSetCount}} 2.5 x 109) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = ( 4.5 x 1010) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = 7.5 x 10-14 g. The mass of 2.5 x 109 molecules of H2O is 7.5 x 10-14 g. The key to success for this type of problem is paying attention to the subscripts in a chemical formula. AP Environmental Science: Help and Review, AP Environmental Science: Homework Help Resource, NY Regents Exam - Chemistry: Help and Review, Ohio Assessments for Educators - Middle Grades Science (029): Practice & Study Guide, TExES Science 7-12 (236): Practice & Study Guide, High School Chemistry: Homework Help Resource, High School Physical Science: Tutoring Solution, CSET Science Subtest I - General Science (215): Practice & Study Guide, Create an account to start this course today. Add together the molar masses of 2 x hydrogen and 1 x oxygen to find the molar mass of H. Find the molar mass of carbon from the periodic table: 12.011 grams. Log in here for access. What is the composition, in atom percent, of an alloy that consists of a) 6.5 wt% Pb and b) 93.5 wt% Sn? Try refreshing the page, or contact customer support. How many moles of copper are present in a sample of tennantite with a mass of 17450 grams? For example, one mole of water and one mole of sodium chloride contain equal numbers of particles. Helpful Tips for Converting Molecules to Grams. Scientists use moles to count small objects such as atoms and molecules. Plus, get practice tests, quizzes, and personalized coaching to help you An analytical balance measure mass in grams, not moles or number of particles. What happens if I accidentally ground the output of an LDO regulator? Likewise, to convert moles to grams, multiply the number of moles by molar mass/1 mole.
If you write everything out first it might be easier to see how the units cancel. The distance between two continuous functions is a continuous function. For example, in this problem, there were two atoms of hydrogen and one atom of oxygen. Grams to Moles Formula | How to Convert Grams to Moles, How a Molecule's Biological Function is Related to Shape, Mass-to-Mass Stoichiometry | Problems, Conversion & Calculation, Moles to Atoms Formula | Using Avogadro's Number, Avogadro's Law and Molar Volume | Avogadro's Law Formula. The periodic table contains entries for all elements. [closed]. https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542 (accessed July 22, 2022). Use the chemical formula for table salt, NaCl. Retrieved from https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542. It was important that the flask be completely dry before the unknown liquid was added so that water present would not vaporize when the flask was heated. A mole is a unit used in chemistry to count particles. Do I have to consider the molecular mass of the oxygen atom or the diatomic oxygen molecule when determining the empirical formula of an iron oxide? | {{course.flashcardSetCount}} 2.5 x 109) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = ( 4.5 x 1010) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = 7.5 x 10-14 g. The mass of 2.5 x 109 molecules of H2O is 7.5 x 10-14 g. The key to success for this type of problem is paying attention to the subscripts in a chemical formula. AP Environmental Science: Help and Review, AP Environmental Science: Homework Help Resource, NY Regents Exam - Chemistry: Help and Review, Ohio Assessments for Educators - Middle Grades Science (029): Practice & Study Guide, TExES Science 7-12 (236): Practice & Study Guide, High School Chemistry: Homework Help Resource, High School Physical Science: Tutoring Solution, CSET Science Subtest I - General Science (215): Practice & Study Guide, Create an account to start this course today. Add together the molar masses of 2 x hydrogen and 1 x oxygen to find the molar mass of H. Find the molar mass of carbon from the periodic table: 12.011 grams. Log in here for access. What is the composition, in atom percent, of an alloy that consists of a) 6.5 wt% Pb and b) 93.5 wt% Sn? Try refreshing the page, or contact customer support. How many moles of copper are present in a sample of tennantite with a mass of 17450 grams? For example, one mole of water and one mole of sodium chloride contain equal numbers of particles. Helpful Tips for Converting Molecules to Grams. Scientists use moles to count small objects such as atoms and molecules. Plus, get practice tests, quizzes, and personalized coaching to help you An analytical balance measure mass in grams, not moles or number of particles. What happens if I accidentally ground the output of an LDO regulator? Likewise, to convert moles to grams, multiply the number of moles by molar mass/1 mole.  The atomic mass usually appears below the element symbol in a periodic table entry. Also, it is much more practical to measure a mole of atoms or molecules than to manipulate individual particles. 6.022 x 1023 is the number of items in a mole, with this constant being named Avogadro's number . Using the mole, scientists can work with microscopic particles using real-world quantities measured in grams. Nissa has a masters degree in chemistry and has taught high school science and college level chemistry. Can a timeseries with a clear trend be considered stationary? 94 lessons, {{courseNav.course.topics.length}} chapters | Chemists physically measure the mass of substances using an analytical balance. Already registered? This is why scientists need a way to know how to convert from grams to moles. succeed. These entries provide the atomic number and atomic mass of each element. A mole of atoms means 6.022 x 1023 atoms.
The atomic mass usually appears below the element symbol in a periodic table entry. Also, it is much more practical to measure a mole of atoms or molecules than to manipulate individual particles. 6.022 x 1023 is the number of items in a mole, with this constant being named Avogadro's number . Using the mole, scientists can work with microscopic particles using real-world quantities measured in grams. Nissa has a masters degree in chemistry and has taught high school science and college level chemistry. Can a timeseries with a clear trend be considered stationary? 94 lessons, {{courseNav.course.topics.length}} chapters | Chemists physically measure the mass of substances using an analytical balance. Already registered? This is why scientists need a way to know how to convert from grams to moles. succeed. These entries provide the atomic number and atomic mass of each element. A mole of atoms means 6.022 x 1023 atoms.
Fireworks In Shreveport Tonight 2022,
Bradley Fire Department Chief,
Theater Step Lighting,
Wrestlemania 33 Match Card Results,
Mass Production Tailoring,
 copyright 2003-2022 Study.com. Amount of substance of a molecule in a solute the same as amount of substance of constituent elements? Write a comment if there's any ambiguity. 8 chapters | Why do the displayed ticks from a Plot of a function not match the ones extracted through Charting`FindTicks in this case? A typical single drop of liquid water has a vo, Working Scholars Bringing Tuition-Free College to the Community. Given the number of moles in a sample, use its molar mass to determine how many grams are present. Stoichiometry Calculations: Help & Review, {{courseNav.course.mDynamicIntFields.lessonCount}}, All Teacher Certification Test Prep Courses, Fundamentals of Analytical Chemistry: Help & Review, Mole-to-Mole Ratios and Calculations of a Chemical Equation, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Limiting Reactants & Calculating Excess Reactants, Calculating Reaction Yield and Percentage Yield from a Limiting Reactant, Calculating Percent Composition and Determining Empirical Formulas, Hydrates: Determining the Chemical Formula From Empirical Data, Types of Chemical Reactions: Help & Review, DSST Foundations of Education: Study Guide & Test Prep, High School Physical Science: Help and Review, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Weather and Climate Science: Certificate Program, Introduction to Astronomy: Certificate Program, Introduction to Physical Geology: Help and Review, NY Regents Exam - Chemistry: Tutoring Solution, Landforms, Climates & Ecosystems of the Earth: Patterns & Characteristics, Quiz & Worksheet - Calculating Motion with Kinematic Equations, Quiz & Worksheet - Characteristics & Types of Force, Quiz & Worksheet - The Difference Between Inertia and Mass, Quiz & Worksheet - Distinguishing Between Mass and Weight, Quiz & Worksheet - Describing Motion with Speed and Velocity, TExES Science of Teaching Reading (293): Practice & Study Guide, Understanding the Scientific Methods for Research, Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Quiz & Worksheet - The Death of Washington, Quiz & Worksheet - Aphorisms in The Importance of Being Earnest, Quiz & Worksheet - US Gang Violence Overview, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Positive Behavior Support | PBIS Tips for Teachers, Middle School Physical Science: Tutoring Solution, ORELA Middle Grades Mathematics: Practice & Study Guide, MTEL General Science (64): Practice & Study Guide, American Politics From 1980 to 1992: Tutoring Solution, Quiz & Worksheet - Spanish Practice: Reading about Shopping, Quiz & Worksheet - Regulating Acid Base Balance, Quiz & Worksheet - Infant Perceptual Development, Quiz & Worksheet - Triarchic Theory of Intelligence, Quiz & Worksheet - Movement Through the Small Intestine, The Ku Klux Klan, Eugenics and Nativism: Definition, Movement & Social Reactions, Online Science Lessons to Use for School Closures, Tech and Engineering - Questions & Answers, Health and Medicine - Questions & Answers, In a chemical reaction 2 mol of substance A react to produce exactly 3 mol of substance B. Basically the premise behind my approach is abusing conversion factors to get from atoms to grams. Scientists use the mole for convenience when measuring matter.
copyright 2003-2022 Study.com. Amount of substance of a molecule in a solute the same as amount of substance of constituent elements? Write a comment if there's any ambiguity. 8 chapters | Why do the displayed ticks from a Plot of a function not match the ones extracted through Charting`FindTicks in this case? A typical single drop of liquid water has a vo, Working Scholars Bringing Tuition-Free College to the Community. Given the number of moles in a sample, use its molar mass to determine how many grams are present. Stoichiometry Calculations: Help & Review, {{courseNav.course.mDynamicIntFields.lessonCount}}, All Teacher Certification Test Prep Courses, Fundamentals of Analytical Chemistry: Help & Review, Mole-to-Mole Ratios and Calculations of a Chemical Equation, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Limiting Reactants & Calculating Excess Reactants, Calculating Reaction Yield and Percentage Yield from a Limiting Reactant, Calculating Percent Composition and Determining Empirical Formulas, Hydrates: Determining the Chemical Formula From Empirical Data, Types of Chemical Reactions: Help & Review, DSST Foundations of Education: Study Guide & Test Prep, High School Physical Science: Help and Review, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Weather and Climate Science: Certificate Program, Introduction to Astronomy: Certificate Program, Introduction to Physical Geology: Help and Review, NY Regents Exam - Chemistry: Tutoring Solution, Landforms, Climates & Ecosystems of the Earth: Patterns & Characteristics, Quiz & Worksheet - Calculating Motion with Kinematic Equations, Quiz & Worksheet - Characteristics & Types of Force, Quiz & Worksheet - The Difference Between Inertia and Mass, Quiz & Worksheet - Distinguishing Between Mass and Weight, Quiz & Worksheet - Describing Motion with Speed and Velocity, TExES Science of Teaching Reading (293): Practice & Study Guide, Understanding the Scientific Methods for Research, Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Quiz & Worksheet - The Death of Washington, Quiz & Worksheet - Aphorisms in The Importance of Being Earnest, Quiz & Worksheet - US Gang Violence Overview, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Positive Behavior Support | PBIS Tips for Teachers, Middle School Physical Science: Tutoring Solution, ORELA Middle Grades Mathematics: Practice & Study Guide, MTEL General Science (64): Practice & Study Guide, American Politics From 1980 to 1992: Tutoring Solution, Quiz & Worksheet - Spanish Practice: Reading about Shopping, Quiz & Worksheet - Regulating Acid Base Balance, Quiz & Worksheet - Infant Perceptual Development, Quiz & Worksheet - Triarchic Theory of Intelligence, Quiz & Worksheet - Movement Through the Small Intestine, The Ku Klux Klan, Eugenics and Nativism: Definition, Movement & Social Reactions, Online Science Lessons to Use for School Closures, Tech and Engineering - Questions & Answers, Health and Medicine - Questions & Answers, In a chemical reaction 2 mol of substance A react to produce exactly 3 mol of substance B. Basically the premise behind my approach is abusing conversion factors to get from atoms to grams. Scientists use the mole for convenience when measuring matter.  The atomic number is the number of protons in each atom of an element. The atomic mass represents the average total mass of one atom of an element. All rights reserved. The mole is a convenient method to deal with substances because of the one-to-one conversion between amus and grams. $$100.0 \, g \, C\, \times \, \frac{1\, mol\, C\, }{12.011\, g\, C} = 8.326 \, mol \, C $$. A chemist requires 0.912 mol Na2CO3, for a reaction. The mass of one atom of oxygen is 15.999 amus. To unlock this lesson you must be a Study.com Member. Limiting Reactant Formula & Examples | What is a Limiting Reactant? How many grams of solute are present in 845 mL of 0.530 M KBr? ThoughtCo. To convert from moles to grams, use the following formula: $$moles\, of\, substance\, \times \, \frac{molar\, mass \, of\, substance}{1 \,mole}\, = \, grams\, of\, substance $$, $$5 \, mol\, S \, \times \frac{32.06\, g \, S}{1 \, mol}\, = \, 160.3\, g\, S $$, $$(6 \, \times\, 12.011 \, g\, C)\, + \, (12\, \times\, 1.008\, g\, H)\, + (6\, \times\, 15.999\, g \, O)\, = \, 180.16 \, g/mol \, C_{6}H_{12}O_{6} $$, $$2\, mol \, C_{6}H_{12}O_{6} \, \times \frac{180.16\, g\, C_{6}H_{12}O_{6} }{1\, mol } \, = \, 360.32 \, g \, C_{6}H_{12}O_{6} $$. Enrolling in a course lets you earn progress by passing quizzes and exams. Find the molar mass of oxygen from the periodic table: 15.999 grams. Next, multiply the molar masses of these elements by the subscript following them in the compound' formula. She has taught science courses at the high school, college, and graduate levels. One amu is approximately the mass of one proton or neutron. In addition, using moles ensures that comparisons are being made between equal numbers of atoms or molecules. To find the molar mass of a compound, refer to the chemical formula of the compound. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." The sum is the molar mass of sodium chloride: Use the chemical formula for magnesium sulfate (MgSO. You have to guess, how much full formulas correspond to this number of formula portions.
The atomic number is the number of protons in each atom of an element. The atomic mass represents the average total mass of one atom of an element. All rights reserved. The mole is a convenient method to deal with substances because of the one-to-one conversion between amus and grams. $$100.0 \, g \, C\, \times \, \frac{1\, mol\, C\, }{12.011\, g\, C} = 8.326 \, mol \, C $$. A chemist requires 0.912 mol Na2CO3, for a reaction. The mass of one atom of oxygen is 15.999 amus. To unlock this lesson you must be a Study.com Member. Limiting Reactant Formula & Examples | What is a Limiting Reactant? How many grams of solute are present in 845 mL of 0.530 M KBr? ThoughtCo. To convert from moles to grams, use the following formula: $$moles\, of\, substance\, \times \, \frac{molar\, mass \, of\, substance}{1 \,mole}\, = \, grams\, of\, substance $$, $$5 \, mol\, S \, \times \frac{32.06\, g \, S}{1 \, mol}\, = \, 160.3\, g\, S $$, $$(6 \, \times\, 12.011 \, g\, C)\, + \, (12\, \times\, 1.008\, g\, H)\, + (6\, \times\, 15.999\, g \, O)\, = \, 180.16 \, g/mol \, C_{6}H_{12}O_{6} $$, $$2\, mol \, C_{6}H_{12}O_{6} \, \times \frac{180.16\, g\, C_{6}H_{12}O_{6} }{1\, mol } \, = \, 360.32 \, g \, C_{6}H_{12}O_{6} $$. Enrolling in a course lets you earn progress by passing quizzes and exams. Find the molar mass of oxygen from the periodic table: 15.999 grams. Next, multiply the molar masses of these elements by the subscript following them in the compound' formula. She has taught science courses at the high school, college, and graduate levels. One amu is approximately the mass of one proton or neutron. In addition, using moles ensures that comparisons are being made between equal numbers of atoms or molecules. To find the molar mass of a compound, refer to the chemical formula of the compound. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." The sum is the molar mass of sodium chloride: Use the chemical formula for magnesium sulfate (MgSO. You have to guess, how much full formulas correspond to this number of formula portions.  Is there a suffix that means "like", or "resembling"? Add up the molar masses of these elements, making sure to multiply the molar mass of each element by the subscript following it in the formula. Announcing the Stacks Editor Beta release! What is the mass in grams of a sample of $\ce{Fe2(SO4)3}$ that The sum is the number of grams in one mole of the compound. The grams to moles conversion uses a substance's molar mass. The average atomic mass of oxygen is 15.999 amus. Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? Use the periodic table to find the substance's molar mass when making conversions between grams to moles and moles to grams. Lewis Structures | Overview, Structural Formula & Examples. To convert moles to grams, use this conversion factor: To convert grams to moles, use this conversion factor. rev2022.7.21.42639. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." Helmenstine, Anne Marie, Ph.D. (2020, August 25). See examples of how to find moles from grams. I'm confused about how to use only the quantity of sulfate ions to find the mass in grams of the whole sample, given it's grams per mole. $$22.990 \, g\, Na \, + \, 35.45\, g \, Cl \, = \, 58.44 \, g/mol \, NaCl $$, $$10 \, g \, NaCl\, \times \, \frac{1 \, mol\, NaCl }{58.44 \, g \, NaCl} = 0.17 \, mol\, NaCl $$, $$24.305\, g\, Mg \, + \, 32.06 \, g\, S \, + \, (4\, \times \, (15.999\, g \, O))\, = \, 120.4 \, g/mol\, MgSO_{4} $$, $$1500\, g\, MgSO_{4}\, \times\frac{1\, mole \, MgSO_{4}}{120.4\, g \, MgSO_{4} } \, =\, 12\, mol\, MgSO_{4} $$. Are propositional atoms recoverable from this Boolean algebra structure? For instance, the atomic number of oxygen is 8, and its atomic mass is 15.999. How to Convert Grams to Moles and Moles to Grams, Avogadro's Number Example Chemistry Problem - Water in a Snowflake, Avogadro's Number Example Chemistry Problem, Calculate Simplest Formula From Percent Composition, Calculating the Number of Atoms and Molecules in a Drop of Water, How to Calculate Limiting Reactant and Theoretical Yield, Theoretical Yield Definition in Chemistry. The molar mass is the same numeric value but in grams instead of amus. Degrees: BS, Computing & Artificial Intelligence, University of Sussex, M.Ed, Science Curriculum & Instruction, University of Missouri. Here is an illustration of how to find moles when given the mass in grams of a substance: $$100.0 \, g \, Li\, \times \, \frac{1\, mol\, Li\, }{6.938\, g\, Li} = 14.41 \, mol \, Li $$. Use the formula of the compound to do this. To find the molar mass of a simple element, look at the periodic table to get the element's atomic mass. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Obviously. So, the molar mass of oxygen is 15.999 grams. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. How do I ask homework questions on Chemistry Stack Exchange? 2A --> 3B How many molecules of substance B are produced when 27.1g of substance A reacts? Different substances have different molar masses. Atomic mass is the average mass of one atom of an element. Calculate the number of mmoles of 1-butanol, sulfuric acid, and sodium bromide. Use the periodic table to find the molar mass of sulfur: 32.06 g/mol. Precipitation Reaction Formula & Formation | What is Precipitation Reaction? How many moles of each compound are contained in a single dose? This value, 6.022 x 1023, is called Avogadro's number. How would you go about solving a question like that? One dose of an antacid contains 870. mg each of Mg(OH)2 and Al(OH)3. The molar mass of an element in grams is the same as the numeric value of the mass of one atom of that element in amus. Learn how to convert grams to moles and moles to grams, including the formula to convert grams to moles. of $\ce{Fe2(SO4)3}$ is $399.91 \, \text{g}/\text{mol}$. Ideal Gas Law Formula & Calculations | Pressure, Temperature & Volume, Percent Yield Formula | How to Calculate Yield, Drawing Isomers of Organic Molecules: Practice Problems, Aluminum Chloride Formula | AlCL3 Decomposition & Lewis Structure. You can then use the given information to solve for grams of ferric sulfate. The formula $\ce{Fe2(SO4)3}$ tells us that 3 molecules of $\ce{SO4^{2-}}$ exist for every $\ce{Fe2(SO4)3}$ molecule. contains $3.59 \times 10^{23}$ sulfate ions, $\ce{SO4^{2}}$ ? A dozen eggs means twelve eggs, whereas a gross of pencils means one hundred and forty-four pencils. Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively. Create your account. All other trademarks and copyrights are the property of their respective owners. Tennantite is an ore of copper with the formula Cu_12As_4S_13. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. An error occurred trying to load this video. The mass in grams of one mole of a substance is called its.
Is there a suffix that means "like", or "resembling"? Add up the molar masses of these elements, making sure to multiply the molar mass of each element by the subscript following it in the formula. Announcing the Stacks Editor Beta release! What is the mass in grams of a sample of $\ce{Fe2(SO4)3}$ that The sum is the number of grams in one mole of the compound. The grams to moles conversion uses a substance's molar mass. The average atomic mass of oxygen is 15.999 amus. Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? Use the periodic table to find the substance's molar mass when making conversions between grams to moles and moles to grams. Lewis Structures | Overview, Structural Formula & Examples. To convert moles to grams, use this conversion factor: To convert grams to moles, use this conversion factor. rev2022.7.21.42639. Helmenstine, Anne Marie, Ph.D. "Use Avogadro's Number to Convert Molecules to Grams." Helmenstine, Anne Marie, Ph.D. (2020, August 25). See examples of how to find moles from grams. I'm confused about how to use only the quantity of sulfate ions to find the mass in grams of the whole sample, given it's grams per mole. $$22.990 \, g\, Na \, + \, 35.45\, g \, Cl \, = \, 58.44 \, g/mol \, NaCl $$, $$10 \, g \, NaCl\, \times \, \frac{1 \, mol\, NaCl }{58.44 \, g \, NaCl} = 0.17 \, mol\, NaCl $$, $$24.305\, g\, Mg \, + \, 32.06 \, g\, S \, + \, (4\, \times \, (15.999\, g \, O))\, = \, 120.4 \, g/mol\, MgSO_{4} $$, $$1500\, g\, MgSO_{4}\, \times\frac{1\, mole \, MgSO_{4}}{120.4\, g \, MgSO_{4} } \, =\, 12\, mol\, MgSO_{4} $$. Are propositional atoms recoverable from this Boolean algebra structure? For instance, the atomic number of oxygen is 8, and its atomic mass is 15.999. How to Convert Grams to Moles and Moles to Grams, Avogadro's Number Example Chemistry Problem - Water in a Snowflake, Avogadro's Number Example Chemistry Problem, Calculate Simplest Formula From Percent Composition, Calculating the Number of Atoms and Molecules in a Drop of Water, How to Calculate Limiting Reactant and Theoretical Yield, Theoretical Yield Definition in Chemistry. The molar mass is the same numeric value but in grams instead of amus. Degrees: BS, Computing & Artificial Intelligence, University of Sussex, M.Ed, Science Curriculum & Instruction, University of Missouri. Here is an illustration of how to find moles when given the mass in grams of a substance: $$100.0 \, g \, Li\, \times \, \frac{1\, mol\, Li\, }{6.938\, g\, Li} = 14.41 \, mol \, Li $$. Use the formula of the compound to do this. To find the molar mass of a simple element, look at the periodic table to get the element's atomic mass. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Obviously. So, the molar mass of oxygen is 15.999 grams. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. How do I ask homework questions on Chemistry Stack Exchange? 2A --> 3B How many molecules of substance B are produced when 27.1g of substance A reacts? Different substances have different molar masses. Atomic mass is the average mass of one atom of an element. Calculate the number of mmoles of 1-butanol, sulfuric acid, and sodium bromide. Use the periodic table to find the molar mass of sulfur: 32.06 g/mol. Precipitation Reaction Formula & Formation | What is Precipitation Reaction? How many moles of each compound are contained in a single dose? This value, 6.022 x 1023, is called Avogadro's number. How would you go about solving a question like that? One dose of an antacid contains 870. mg each of Mg(OH)2 and Al(OH)3. The molar mass of an element in grams is the same as the numeric value of the mass of one atom of that element in amus. Learn how to convert grams to moles and moles to grams, including the formula to convert grams to moles. of $\ce{Fe2(SO4)3}$ is $399.91 \, \text{g}/\text{mol}$. Ideal Gas Law Formula & Calculations | Pressure, Temperature & Volume, Percent Yield Formula | How to Calculate Yield, Drawing Isomers of Organic Molecules: Practice Problems, Aluminum Chloride Formula | AlCL3 Decomposition & Lewis Structure. You can then use the given information to solve for grams of ferric sulfate. The formula $\ce{Fe2(SO4)3}$ tells us that 3 molecules of $\ce{SO4^{2-}}$ exist for every $\ce{Fe2(SO4)3}$ molecule. contains $3.59 \times 10^{23}$ sulfate ions, $\ce{SO4^{2}}$ ? A dozen eggs means twelve eggs, whereas a gross of pencils means one hundred and forty-four pencils. Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively. Create your account. All other trademarks and copyrights are the property of their respective owners. Tennantite is an ore of copper with the formula Cu_12As_4S_13. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. An error occurred trying to load this video. The mass in grams of one mole of a substance is called its.  An analytical balance measures grams; it cannot count moles, amus, or the number of particles in a sample. How would you find the mass in grams, if you only know the number of particles of a portion of the formula? Refer to the periodic table to find and add up the molar masses of magnesium (Mg), sulfur (S), and 4 x oxygen (O). The sum is the molar mass of magnesium sulfate. Homework questions must demonstrate some effort to understand the underlying concepts. While working for two years in cancer research, she published articles in academic journals. As a member, you'll also get unlimited access to over 84,000 Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer, What Is a Conversion Factor? Using grams in calculations instead of moles would be unwieldy and confusing. Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. Kathryn Seifert Antonelli is a teacher certified in Biology, Chemistry, and General Science. How many grams does this correspond to? As a unit of measurement, the mole is like a dozen or a gross. (instead of occupation of Japan, occupied Japan or Occupation-era Japan), Scientifically plausible way to sink a landmass. What's the use of 100k resistors in this schematic.
An analytical balance measures grams; it cannot count moles, amus, or the number of particles in a sample. How would you find the mass in grams, if you only know the number of particles of a portion of the formula? Refer to the periodic table to find and add up the molar masses of magnesium (Mg), sulfur (S), and 4 x oxygen (O). The sum is the molar mass of magnesium sulfate. Homework questions must demonstrate some effort to understand the underlying concepts. While working for two years in cancer research, she published articles in academic journals. As a member, you'll also get unlimited access to over 84,000 Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples, Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer, What Is a Conversion Factor? Using grams in calculations instead of moles would be unwieldy and confusing. Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. Kathryn Seifert Antonelli is a teacher certified in Biology, Chemistry, and General Science. How many grams does this correspond to? As a unit of measurement, the mole is like a dozen or a gross. (instead of occupation of Japan, occupied Japan or Occupation-era Japan), Scientifically plausible way to sink a landmass. What's the use of 100k resistors in this schematic.  What advantages does the mole have over counting large numbers using SI prefixes? If you're getting the incorrect answer for this type of problem, the usual cause is having the number of atoms wrong.
What advantages does the mole have over counting large numbers using SI prefixes? If you're getting the incorrect answer for this type of problem, the usual cause is having the number of atoms wrong.  If you write everything out first it might be easier to see how the units cancel. The distance between two continuous functions is a continuous function. For example, in this problem, there were two atoms of hydrogen and one atom of oxygen. Grams to Moles Formula | How to Convert Grams to Moles, How a Molecule's Biological Function is Related to Shape, Mass-to-Mass Stoichiometry | Problems, Conversion & Calculation, Moles to Atoms Formula | Using Avogadro's Number, Avogadro's Law and Molar Volume | Avogadro's Law Formula. The periodic table contains entries for all elements. [closed]. https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542 (accessed July 22, 2022). Use the chemical formula for table salt, NaCl. Retrieved from https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542. It was important that the flask be completely dry before the unknown liquid was added so that water present would not vaporize when the flask was heated. A mole is a unit used in chemistry to count particles. Do I have to consider the molecular mass of the oxygen atom or the diatomic oxygen molecule when determining the empirical formula of an iron oxide? | {{course.flashcardSetCount}} 2.5 x 109) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = ( 4.5 x 1010) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = 7.5 x 10-14 g. The mass of 2.5 x 109 molecules of H2O is 7.5 x 10-14 g. The key to success for this type of problem is paying attention to the subscripts in a chemical formula. AP Environmental Science: Help and Review, AP Environmental Science: Homework Help Resource, NY Regents Exam - Chemistry: Help and Review, Ohio Assessments for Educators - Middle Grades Science (029): Practice & Study Guide, TExES Science 7-12 (236): Practice & Study Guide, High School Chemistry: Homework Help Resource, High School Physical Science: Tutoring Solution, CSET Science Subtest I - General Science (215): Practice & Study Guide, Create an account to start this course today. Add together the molar masses of 2 x hydrogen and 1 x oxygen to find the molar mass of H. Find the molar mass of carbon from the periodic table: 12.011 grams. Log in here for access. What is the composition, in atom percent, of an alloy that consists of a) 6.5 wt% Pb and b) 93.5 wt% Sn? Try refreshing the page, or contact customer support. How many moles of copper are present in a sample of tennantite with a mass of 17450 grams? For example, one mole of water and one mole of sodium chloride contain equal numbers of particles. Helpful Tips for Converting Molecules to Grams. Scientists use moles to count small objects such as atoms and molecules. Plus, get practice tests, quizzes, and personalized coaching to help you An analytical balance measure mass in grams, not moles or number of particles. What happens if I accidentally ground the output of an LDO regulator? Likewise, to convert moles to grams, multiply the number of moles by molar mass/1 mole.
If you write everything out first it might be easier to see how the units cancel. The distance between two continuous functions is a continuous function. For example, in this problem, there were two atoms of hydrogen and one atom of oxygen. Grams to Moles Formula | How to Convert Grams to Moles, How a Molecule's Biological Function is Related to Shape, Mass-to-Mass Stoichiometry | Problems, Conversion & Calculation, Moles to Atoms Formula | Using Avogadro's Number, Avogadro's Law and Molar Volume | Avogadro's Law Formula. The periodic table contains entries for all elements. [closed]. https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542 (accessed July 22, 2022). Use the chemical formula for table salt, NaCl. Retrieved from https://www.thoughtco.com/avogadros-number-chemistry-problem-example-609542. It was important that the flask be completely dry before the unknown liquid was added so that water present would not vaporize when the flask was heated. A mole is a unit used in chemistry to count particles. Do I have to consider the molecular mass of the oxygen atom or the diatomic oxygen molecule when determining the empirical formula of an iron oxide? | {{course.flashcardSetCount}} 2.5 x 109) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = ( 4.5 x 1010) / 6.022 x 1023 H2O moleculesmass of 2.5 x 109 molecules of H2O = 7.5 x 10-14 g. The mass of 2.5 x 109 molecules of H2O is 7.5 x 10-14 g. The key to success for this type of problem is paying attention to the subscripts in a chemical formula. AP Environmental Science: Help and Review, AP Environmental Science: Homework Help Resource, NY Regents Exam - Chemistry: Help and Review, Ohio Assessments for Educators - Middle Grades Science (029): Practice & Study Guide, TExES Science 7-12 (236): Practice & Study Guide, High School Chemistry: Homework Help Resource, High School Physical Science: Tutoring Solution, CSET Science Subtest I - General Science (215): Practice & Study Guide, Create an account to start this course today. Add together the molar masses of 2 x hydrogen and 1 x oxygen to find the molar mass of H. Find the molar mass of carbon from the periodic table: 12.011 grams. Log in here for access. What is the composition, in atom percent, of an alloy that consists of a) 6.5 wt% Pb and b) 93.5 wt% Sn? Try refreshing the page, or contact customer support. How many moles of copper are present in a sample of tennantite with a mass of 17450 grams? For example, one mole of water and one mole of sodium chloride contain equal numbers of particles. Helpful Tips for Converting Molecules to Grams. Scientists use moles to count small objects such as atoms and molecules. Plus, get practice tests, quizzes, and personalized coaching to help you An analytical balance measure mass in grams, not moles or number of particles. What happens if I accidentally ground the output of an LDO regulator? Likewise, to convert moles to grams, multiply the number of moles by molar mass/1 mole.  The atomic mass usually appears below the element symbol in a periodic table entry. Also, it is much more practical to measure a mole of atoms or molecules than to manipulate individual particles. 6.022 x 1023 is the number of items in a mole, with this constant being named Avogadro's number . Using the mole, scientists can work with microscopic particles using real-world quantities measured in grams. Nissa has a masters degree in chemistry and has taught high school science and college level chemistry. Can a timeseries with a clear trend be considered stationary? 94 lessons, {{courseNav.course.topics.length}} chapters | Chemists physically measure the mass of substances using an analytical balance. Already registered? This is why scientists need a way to know how to convert from grams to moles. succeed. These entries provide the atomic number and atomic mass of each element. A mole of atoms means 6.022 x 1023 atoms.
The atomic mass usually appears below the element symbol in a periodic table entry. Also, it is much more practical to measure a mole of atoms or molecules than to manipulate individual particles. 6.022 x 1023 is the number of items in a mole, with this constant being named Avogadro's number . Using the mole, scientists can work with microscopic particles using real-world quantities measured in grams. Nissa has a masters degree in chemistry and has taught high school science and college level chemistry. Can a timeseries with a clear trend be considered stationary? 94 lessons, {{courseNav.course.topics.length}} chapters | Chemists physically measure the mass of substances using an analytical balance. Already registered? This is why scientists need a way to know how to convert from grams to moles. succeed. These entries provide the atomic number and atomic mass of each element. A mole of atoms means 6.022 x 1023 atoms.